
A comparison of SARS-CoV-2 antibody profiles in the elderly and young
In a recent Scientific Reports study, researchers comparatively evaluate antibody titers after dual vaccination with the Pfizer-BioNTech BNT162b2 messenger ribonucleic acid (mRNA) vaccine against coronavirus 2 (SARS-CoV-2) of severe acute respiratory syndrome among very old older people. aged 90 and a younger population aged between 23 and 69.
Previous studies have reported a progressive decline in immune system responses and remodeling with advancing age, causing the effectiveness of SARS-CoV-2 vaccines to vary with age. In particular, elderly patients are at greater risk of severe illness from coronavirus 2019 (COVID-19); however, serological data on elderly people over 90 years of age after vaccination against COVID-19 are limited.
About the study
In the present cross-sectional study, the researchers assessed anti-SARS-CoV-2 humoral immune responses in very elderly people and compared their findings with data from a younger population aged 23 to 69 years after the BNT162b2 vaccination in Italy.
Data were obtained from post-vaccination serological reports of anti-SARS-CoV-2 antibodies in the very old and younger/general population. These samples were collected as part of the COVIDIAG-NOSTIX project between December 2020 and September 2021.
The younger population consisted of healthcare workers who were recruited in a COVID-19 vaccination campaign in Italy. Comparatively, the very large population was made up of people receiving home assistance from the Cooperative of the Health Operator Association (OSA) for the purposes of medication and rehabilitation.
Sera were obtained from study participants after six months of double BNT162b2 mRNA vaccination. The elderly cohort also completed the rapid antibody test during serum sample collection.
Antibodies against the SARS-CoV-2 spike protein receptor-binding domain (RBD) (S) and nucleocapsid protein (N) were assessed by electrochemiluminescence immunoassays (ECLIA). Rapid antibody testing involved chemiluminescence assays (CLIA) to detect anti-SARS-CoV-2 S immunoglobulin G (IgG).
Chromatographic assay-based rapid antibody tests were also performed to detect anti-S and anti-N IgM and IgG titers. These tests were completed in 10 minutes and allowed the researchers to get immediate results for very old people.
Results of the study
The very old and youngest population included 97 and 1,114 individuals, respectively. Follow-up periods and proportions of individuals with anti-SARS-CoV-2 N seropositivity of 8–9% and women were similar among study participants.
Significant differences in anti-SARS-CoV-2 S titers were observed between anti-N seropositive and anti-N seronegative participants, with mean values of 2,500 U/mL and 587 U/mL in the younger population, respectively. Corresponding values of 2,500 U/ml and 65 U/ml were reported in the very old population, respectively.
Considering only seronegative individuals, a significant decrease was observed between both groups, with a lower humoral response observed in the elderly. Sex-based immune differences were only observed in the younger population.
More than 60% of HIV-positive cases among the youngest group were women. In comparison, none of the male participants showed HIV-positivity in the very large group, which included only seven women. Therefore, comparisons could only be made between women. To this end, no significant differences were observed between the youngest and very old participants.
Anti-N seropositivity and age were the most important factors in determining anti-SARS-CoV-2 S antibody levels, followed by sex, which produced significant but limited effects on overall outcomes. Among very old participants, identical differences were observed between anti-SARS-CoV-2 N seropositive and seronegative individuals, with mean values of 2080 U/mL and 42.5 AU/mL between anti-N seropositive and seronegative individuals, respectively .
Rapid antibody tests were able to detect anti-S IgG antibodies, with positive serum antibody titers greater than 197 AU/ml in the Roche Elecsys test with a specificity and sensitivity of 83% and 100%, respectively . In addition, correlations were observed between good reports of serological tests and positive immune levels in rapid antibody tests with mean values of 3263 AU/mL, 899 AU/mL, and 809 AU/mL for strongly positive, positive, and slightly positive reports respectively.
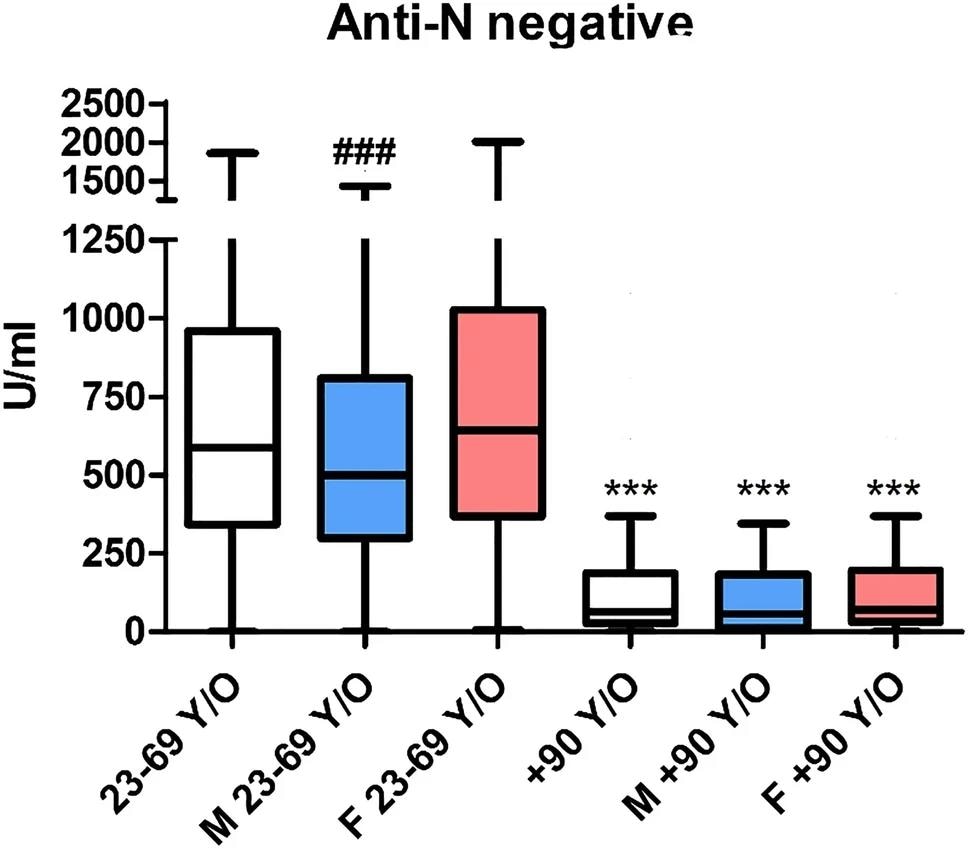
Anti-S antibody titer (from Roche Elecsys SARS-CoV-2-S) in individuals seronegative for anti-N. Blue box (M) indicates male subjects and red (F) indicates female subjects. ***p < 0.001 versus the corresponding color in the 23–69 year cohort; ### p <0.001 versus women in the same cohort.
Conclusions
The study results show that very elderly people obtained detectable anti-SARS-CoV-2 S titers after the second BNT162b2 vaccination. This response was mainly observed among anti-N seropositive individuals who had a previous history of COVID-19. In this group, anti-N titers were comparable to those in the younger cohort.
Similarly, the anti-N seronegative elderly had less anti-S antibodies compared to the younger cohort. Thus, SARS-CoV-2-specific antibody responses varied as a function of anti-N serostatus, individual age, and sex among the very elderly.
The present study was the first of its kind to provide post-vaccination data among people over 90 years of age. These findings could potentially affect the organization of the COVID-19 vaccination campaign, including the administration of booster doses and the prioritization of vaccine recipients.
In addition, anti-SARS-CoV-2 antibody titers could be used as supportive indices to establish the need for additional doses of vaccine, especially among the elderly population, whose immune responses to infections may not be as efficient as those of immunocompetent individuals with antecedents. of COVID-19.
Journal reference
Tomaiuolo, R., Di Resta, C., Viganò, M. et al. (2022). Six months of SARS-CoV-2 serology in a cohort of mRNA-vaccinated subjects over 90 years of age. Scientific Reports 12 (12446).

Comments are closed.